The School of Science is pleased to launch the TCNJ–Novo Nordisk Lecture Series during the 2015-2016 academic year. The series will include four lectures throughout the year, two during the Fall 2015 semester and two during the Spring 2016 semester.
About the TCNJ–Novo Nordisk Lecture Series
 Headquartered in Denmark, Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. The company also has leading positions within haemophilia care, growth hormone therapy, and hormone replacement therapy.
Headquartered in Denmark, Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. The company also has leading positions within haemophilia care, growth hormone therapy, and hormone replacement therapy.
With the company’s U.S. headquarters located in Plainsboro, NJ, Novo Nordisk has long been committed to establishing programs throughout the Garden State that strive to make an impact in our local communities. The Novo Nordisk Lecture Series is an exciting new collaboration with The College of New Jersey that provides TCNJ faculty and students with the unique opportunity to learn and engage in a dialog with company leaders representing a variety of disciplines. Presenters will explore topics that represent the state-of-the-art in their respective fields, including drug discovery and development, clinical trials, and the changing delivery of healthcare in the U.S.
Fall 2015 Lectures
October Lecture – “How a Drug is Born”
Dr. Todd Hobbs and Dr. Henriette Mersebach
Tuesday, October 20, 4:00-5:00 pm, SCP-101
 Speakers:
Speakers:
- Dr. Todd Hobbs, MD; Vice President and Chief Medical Officer for Novo Nordisk in North America
- Dr. Henriette Mersebach, MD, PhD; Executive Director of Clinical Development & Research – Diabetes & Obesity for Novo Nordisk in North America
Tuesday, October 20, 2015
4:00-5:00 pm, Lecture followed by reception
Science Complex, South (Physics Wing), SCP-101
Presentation Overview
The first in a series of lectures to highlight the recent partnership between Novo Nordisk and TCNJ, this lecture will take the audience through the many steps required in the process of drug discovery through approval. Scientific leaders from Novo Nordisk, Inc. in Plainsboro, NJ will share their insights gathered from many years of experience in product approvals to explain how a chemical entity moves from the lab to animal and human studies, full-scale clinical testing, and finally to regulatory approval from the FDA to be marketed as a new pharmaceutical product. The speakers will also highlight the many synergies between areas of scientific study available at TCNJ and the relevance of these areas to the drug development process.
About the Speakers
Todd M. Hobbs, MD
 Todd Hobbs, MD, is Vice President and Chief Medical Officer for Novo Nordisk in North America, where he leads the organization’s focus on the implications of diabetes for the patient, healthcare system and healthcare professionals.
Todd Hobbs, MD, is Vice President and Chief Medical Officer for Novo Nordisk in North America, where he leads the organization’s focus on the implications of diabetes for the patient, healthcare system and healthcare professionals.
Dr. Hobbs provides overall medical guidance to Novo Nordisk’s diabetes- and obesity-related projects. He provides input into the clinical development and life-cycle management strategies for diabetes and obesity, as well as medical input into the R&D pipeline. He is involved with the optimization of relationships with top key opinion leaders and medical societies, and provides guidance to and participates in consultant advisory boards and key patient and professional associations and top thought leaders in diabetes.
Dr. Hobbs began his career at Novo Nordisk in 2004 as a field medical scientific director, then moving to the position of senior medical director, diabetes, in 2010. He led the Medical Affairs activities for all of Novo Nordisk’s current insulin products and devices, as well as supporting future insulin products through strategic and tactical activities.
Prior to working at Novo Nordisk, Dr. Hobbs had established a clinical practice based in Louisville, Kentucky, focusing on the intensive management of patients with diabetes of all ages, and served as chairman of the Medicine Department for a large Regional Medical Center in Kentucky. During this 10-year clinical career, he cared for more than 2,500 adults and children with diabetes, including outpatient and inpatient care, as well as intensive care.
Dr. Hobbs’ has a unique perspective on and personal dedication to the treatment of diabetes: his own experience with the disease began more than 25 years ago when he was diagnosed with type 1 diabetes. His passion for defeating the disease intensified even more with the diagnosis of one of his sons with the same condition at the age of five.
Henriette Mersebach, MD, PhD
 Henriette Mersebach, MD, PhD, is Executive Director of Clinical Development & Research – Diabetes & Obesity for Novo Nordisk in North America. Dr. Mersebach holds the leadership responsibilities for the medical/clinical development team and represents the North American Clinical, Medical and Regulatory aspects in the planning and execution of Novo Nordisk’s global drug development programs. She engages in medical dialogue with regulators, investigators and thought leaders within diabetes and obesity.
Henriette Mersebach, MD, PhD, is Executive Director of Clinical Development & Research – Diabetes & Obesity for Novo Nordisk in North America. Dr. Mersebach holds the leadership responsibilities for the medical/clinical development team and represents the North American Clinical, Medical and Regulatory aspects in the planning and execution of Novo Nordisk’s global drug development programs. She engages in medical dialogue with regulators, investigators and thought leaders within diabetes and obesity.
Dr. Mersebach was trained at the University Hospital of Copenhagen, Denmark, Department of Endocrinology, and obtained her PhD in Endocrinology in 2004. She has a degree in Pharmaceutical Medicine from the University of Basel, Switzerland and an executive leadership education from the Scandinavian International Management Institute in Copenhagen, Denmark.
Dr. Mersebach has performed research in the areas of diabetes, obesity, growth hormone disorders, and general endocrinology. She joined Novo Nordisk A/S in 2005 and has been involved in all phases of clinical development within diabetes & obesity.
November Lecture – “Advancements in Understanding of Bleeding Disorders”
Dr. David Ungar
Friday, November 13, 12:30-1:30pm, SCP-101
- Dr. David Ungar, MD, Director in the BioPharm Medical group for Novo Nordisk in North America
Friday, November 13, 2015
12:30-1:30 pm, Lecture followed by reception
Science Complex, South (Physics Wing), SCP-101
Presentation Overview
The second in a series of lectures to highlight the recent partnership between Novo Nordisk and TCNJ, this lecture will build on the October lecture, which reviewed the drug development process and how drugs move from lab to pharmacy. In lecture 2, Dr. David Ungar, a leader in the Biopharm Medical Group at Novo Nordisk will provide an overview of the clotting cascade, review clinical presentations of patients with abnormalities in clotting factors, and highlight the advancements which have been made in recent years to better address these rare but serious disorders. Dr. Ungar has dedicated his career to research, teaching, and care of children and adults who are affected by these, and other, serious diseases. He looks forward to the opportunity to share this extensive background with the students and faculty at TCNJ.
About the Speaker
David Ungar, MD
 David Ungar, MD, is a director in the BioPharm Medical group for Novo Nordisk in North America, where his focus is on bleeding disorders.
David Ungar, MD, is a director in the BioPharm Medical group for Novo Nordisk in North America, where his focus is on bleeding disorders.
Dr. Ungar provides medical guidance to Novo Nordisk’s projects in hemophilia and other rare bleeding disorders. He provides input into the clinical development and life-cycle management strategies for bleeding disorders, as well as medical input into the R&D pipeline. He is involved with the optimization of relationships with top key opinion leaders and medical societies, and provides guidance to and participates in consultant advisory boards. In addition, he oversees the US conduct of clinical trials for several new compounds for hemophilia.
Dr. Ungar began his career at Novo Nordisk in 2011. Prior to working at Novo Nordisk, Dr. Ungar was on the faculty of the Penn State College of Medicine in Hershey PA where he cared for children and adolescents with hemophilia and other bleeding disorders at the Hemophilia Center of Central Pennsylvania. During his 18 years at Penn State, he served in multiple roles, including Clinical Director of the Division of Pediatric Hematology/Oncology, Vice Chair for Clinical Affairs in the Department of Pediatrics, and Chair of the Institutional Review Board.
Spring 2016 Lectures
March Lecture – “Advancements in Type 1 Diabetes”
Dr. Fannie Smith
Tuesday, March 29, 2016, 4:00-5:00 pm, SCP-101
 Speaker:
Speaker:
- Dr. Fannie Smith, MD, PhD, Medical Director, Diabetes & Obesity, Novo Nordisk in North America
Tuesday, March 29, 2016
4:00-5:00 pm, Lecture followed by reception
Science Complex, South (Physics Wing), SCP-101
Presentation Overview
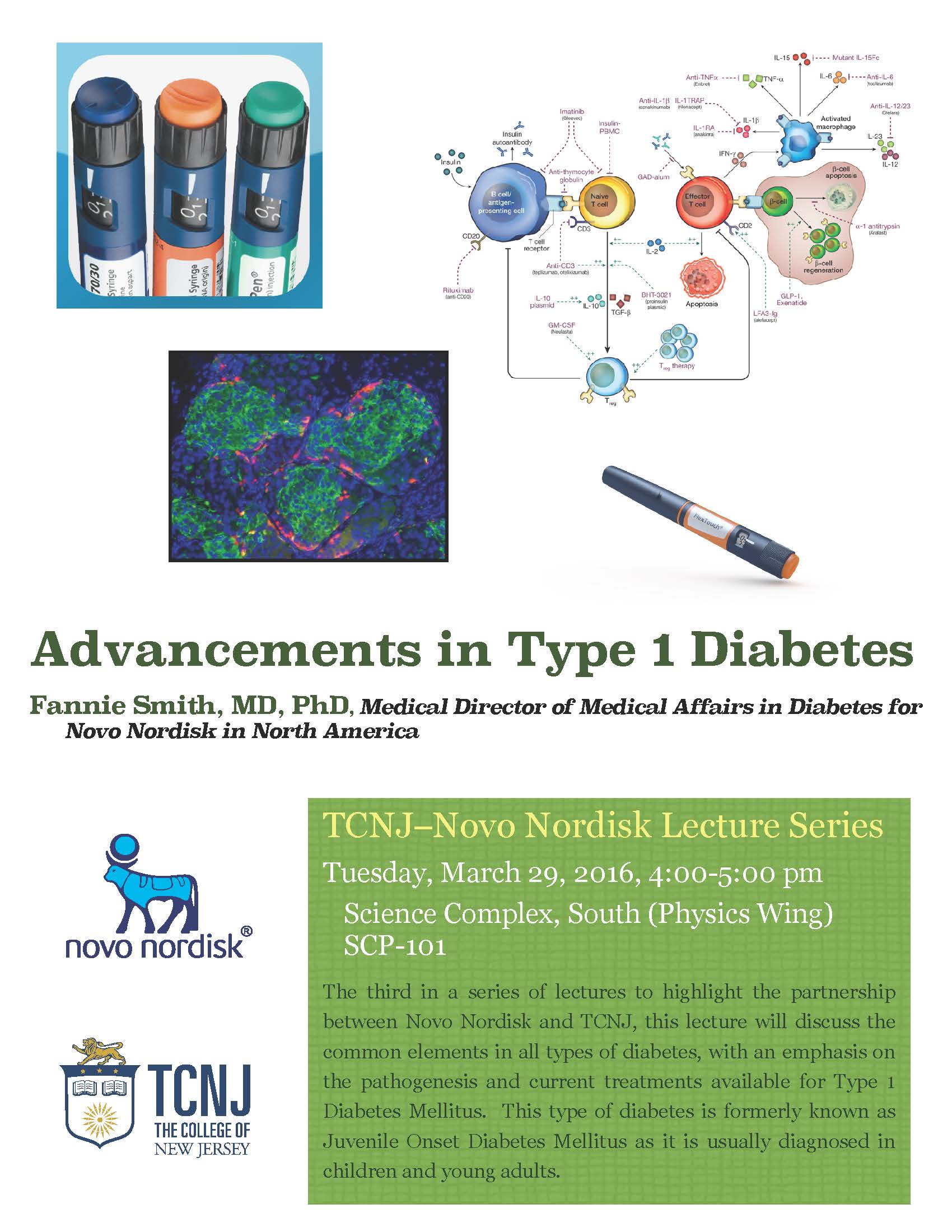
The third in a series of lectures highlighting the partnership between Novo Nordisk and TCNJ. In this lecture, Dr. Fannie Smith, Director of Medical Affairs in Diabetes, will discuss the common elements in all types of diabetes with an emphasis on the pathogenesis and current treatments available for Type 1 Diabetes Mellitus. This type of diabetes is formerly known as Juvenile Onset Diabetes Mellitus as it is usually diagnosed in children and young adults. She will discuss the how biotechnology innovation in insulin pumps and continuous glucose monitoring have changed the treatment and quality of life for individuals living with this chronic disease. The discussion will include information on therapies under development including the artificial pancreas, encapsulating pancreatic islets, and regenerating pancreatic beta cells.
About the Speaker
Fannie Smith, MD, PhD
 Fannie Smith, MD, PhD, is Medical Director of Medical Affairs in Diabetes, Novo Nordisk in North America.
Fannie Smith, MD, PhD, is Medical Director of Medical Affairs in Diabetes, Novo Nordisk in North America.
Dr. Smith has spent a large part of her career in academics, conducting both basic and clinical research in diabetes. Through her work, and in this lecture, she would love to encourage anyone interested in research in diabetes or a medical career to help us find a cure.
Dr. Smith began her career at Novo Nordisk in 2003. Prior to joining Novo Nordisk, she served on the faculty of the University of Texas Medical Branch and served as a clinical associate and researcher at the Joslin Diabetes Center in Boston.
Dr. Smith earned her MD in Medicine at Texas A&M University, her PhD in Cell Biology at Texas Tech University, and her MA and BS in Biology from Sam Houston State University.
April Lecture – “Overview of Incretins and the Science Behind GLP-1”
Dr. Cory Gamble, DO, FACOI, FACE
Tuesday, April 12, 2016, 4:00-5:00 pm, SCP-101
 Speaker:
Speaker:
- Dr. Cory Gamble, DO, FACOI, FACE, Medical Director, Diabetes & Obesity, Novo Nordisk in North America
Tuesday, April 12, 2016
4:00-5:00 pm, Lecture followed by reception
Science Complex, South (Physics Wing), SCP-101
Presentation Overview
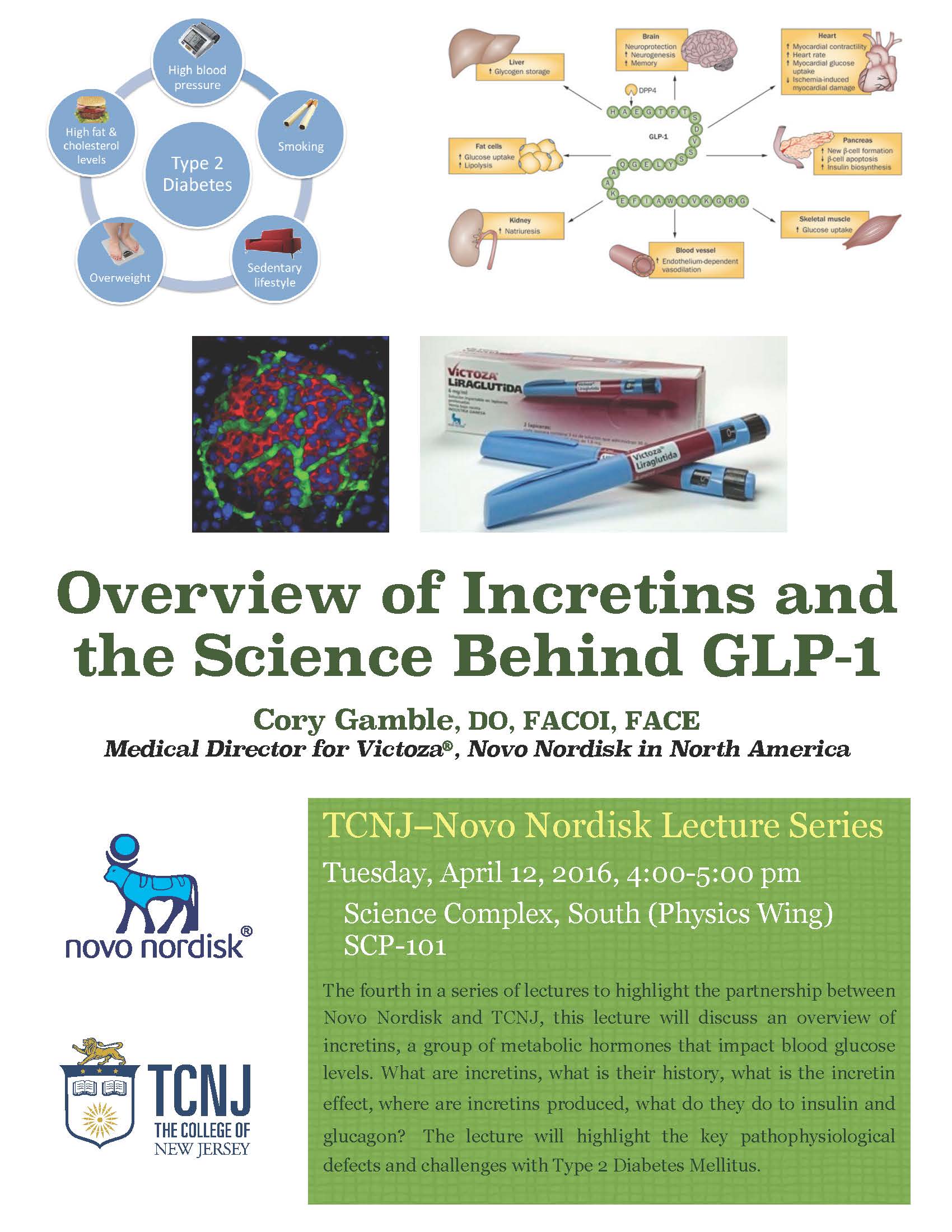
The fourth in a series of lectures highlighting the partnership between Novo Nordisk and TCNJ. In this lecture, Dr. Cory Gamble, Medical Director for Victoza®, will discuss an overview of incretins, a group of metabolic hormones that impact blood glucose levels. In particular, what are incretins, what is their history, what is the incretin effect, where are incretins produced, what do they do to insulin and glucagon? The lecture will highlight the key pathophysiological defects and challenges with Type 2 Diabetes Mellitus. Dr. Gamble will also review of the five currently available GLP-1 receptor agonists, their efficacy, as well as potential safety issues. The discussion will include information about what the future may hold for Entero-insular agonist drugs, including both dual and triple agonists.
About the Speaker
Cory Gamble, DO, FACOI, FACE
 Dr. Cory L Gamble is the U.S. Medical Director for Victoza®. He represents the product in a wide range of areas including but not limited to: life cycle management, labeling, publication planning, medical review, market shaping, Phase IV trial design, and speaker training. He gained 20 years of clinical endocrinology experience after graduating from Oklahoma State University College of Osteopathic Medicine and completion of a residency in Internal Medicine and a Fellowship in Endocrinology, Metabolism, and Hypertension at the University of Oklahoma Health Sciences Center. He earned the George Bean Outstanding Physician Award and was named among the best endocrinologists in Arkansas by a physicians’ poll in the Arkansas Times. Dr. Gamble started his highly successful career at Novo Nordisk as a Medical Scientific Director for the Mississippi Valley Region in 2008. He then served as an Endocrine Medical Liaison for the Memphis district and subsequently as a Regional Medical Scientist for the Southcentral Region and Chairman of the NNI US Research Grants Committee before.
Dr. Cory L Gamble is the U.S. Medical Director for Victoza®. He represents the product in a wide range of areas including but not limited to: life cycle management, labeling, publication planning, medical review, market shaping, Phase IV trial design, and speaker training. He gained 20 years of clinical endocrinology experience after graduating from Oklahoma State University College of Osteopathic Medicine and completion of a residency in Internal Medicine and a Fellowship in Endocrinology, Metabolism, and Hypertension at the University of Oklahoma Health Sciences Center. He earned the George Bean Outstanding Physician Award and was named among the best endocrinologists in Arkansas by a physicians’ poll in the Arkansas Times. Dr. Gamble started his highly successful career at Novo Nordisk as a Medical Scientific Director for the Mississippi Valley Region in 2008. He then served as an Endocrine Medical Liaison for the Memphis district and subsequently as a Regional Medical Scientist for the Southcentral Region and Chairman of the NNI US Research Grants Committee before.
TCNJ–Novo Nordisk Student Prizes
In addition to sponsoring the Lecture Series, Novo Nordisk will be a sponsor of TCNJ’s 2016 Celebration of Student Achievement in a way that directly links student participation in the TCNJ–Novo Nordisk Lecture Series with presentation of the students’ undergraduate research at the campus-wide Celebration.
Three Novo Nordisk Student Prizes, in the form of tuition scholarship support for $2,500 each for the 2016-2017 academic year, will be competitively awarded to student researchers who have attended at least two of the TCNJ–Novo Nordisk lectures during the 2015-2016 academic year and who will be presenting their undergraduate research at the spring 2016 Celebration of Student Achievement.
Student Eligibility and Application
Eligible students will include those in their Sophomore or Junior years. These students should have attended at least two of the Novo Nordisk lectures during the 2015-2016 academic year and will be presenting their undergraduate research at the 2016 Celebration of Student Achievement.
Eligible students will complete an application, which will include (a) demographic and academic information (e.g., academic major, year in the college, grade point average, etc.), (b) an essay on what they learned from participation in the TCNJ–Novo Nordisk lectures and how this connects with their coursework and/or research, (c) an essay on what they have learned from their undergraduate research experience, and (d) an essay describing their aspirations and plans for pursuing a research-based career in the sciences. The three awardees will be competitively selected by a group of TCNJ science faculty members.

